Yuan Wen, Liu Xinrong, Fu Yan. Chemical thermodynamics and chemical kinetics analysis of sandstone dissolution under the action of dry-wet cycles in acid and alkaline environments[J]. Bulletin of Engineering Geology and the Environment, 2019, 78(2): 793-801.
Keywords: Sandstone; Deterioration; Dry–wet cycles; Chemical thermodynamics; Chemical kinetics
Abstract:
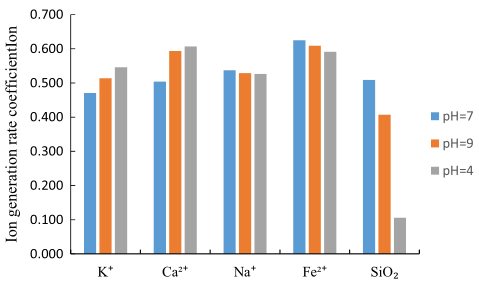
The aim of this paper is to study the mechanism of strength deterioration of sandstone under the action of dry–wet cycles in acid and alkaline environments based on the basic principles of chemical thermodynamics and chemical kinetics. To do that, uniaxial and triaxial tests were conducted on sandstone after different dry–wet cycles in acid and alkaline environments to ascertain its cohesion and internal friction angle, and the relationship between these and the number of dry–wet cycles. On that basis, the variation of the sandstone shear strength with the number of cycles was obtained. Based on the basic principles of chemical thermodynamics, the stability of the main minerals in neutral, alkaline, and acid solutions were determined. After each dry–wet cycle, the concentration of some ions (SiO2, K+, Na+, Ca2+, and Fe2+) in different solutions was measured. The generation rate of each ion was then calculated using chemical kinetics. The results show that the deterioration of sandstone shear strength is most severe in an acid environment, followed by that in an alkaline environment and that in neutral environment. In acid solutions, potash feldspar, albite, calcite, and biotite are unstable. In alkaline solutions, quartz is unstable. The thermodynamic analysis results are consistent with kinetic test results.
Resource:https://link.springer.com/article/10.1007%2Fs10064-017-1162-9